
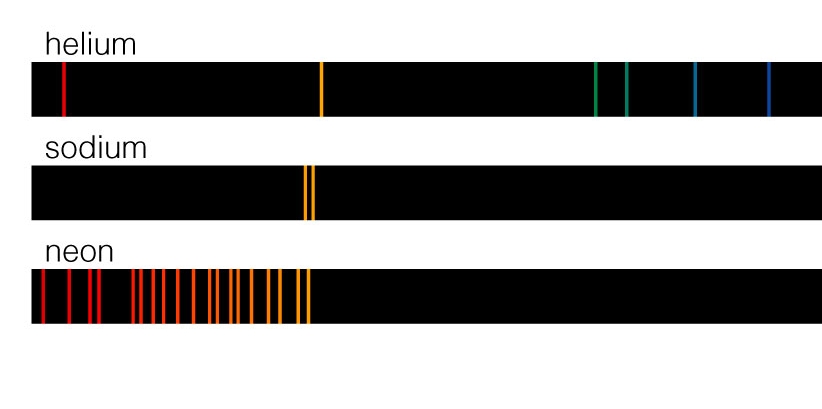
orbital potential energies #V_(2s) ne V_(2p)#, #V_(3s) ne V_(3p) ne V_(3d)#, etc., we can see the following trends: That occurs because having two electrons in helium introduces electron correlation, which splits levels of different angular momentum, because they no longer have spherical symmetry.īeyond that difference, which is readily seen in multi-electron atoms having, e.g. To be fair, I ignored the #2p#, #3p#, #3d#, #4p#, #4d#, and #4f# energy levels, which ARE present AND split away from the #s# levels in helium (but are degenerate in hydrogen), because they are too subtle on the above scale: These energy level gaps are different, and since transitions between them lead to a spectrum, the spectrum is of course also different. Using Excel, and the energy levels of helium given numerically here (estimating the #4s# and #5s#), I've superposed them next to those of hydrogen: Those for helium have no straightforward formula, but are known experimentally. The energy levels of the hydrogen atom are well-known: Well, is it not just a different atom, with more than one electron? The spectrum of helium must be more complex, because now angular momentum becomes a factor to which transitions are allowed it must change by #1# each time.


 0 kommentar(er)
0 kommentar(er)
